Fluorine Bohr Model
1 Ry = e4me 8ϵ2 0h2 = 2.18 × 10 − 18 J. and this simplifies the allowed energies predicted by the Bohr model (Equation 7.4.11) as. En = − (2.18 × 10 − 18)Z2 n2 J = − Z2 n2 Ry. Hence, the energy of the electron in an atom also is quantized. Equation 7.4.12 gives the energies of the electronic states of the hydrogen atom.
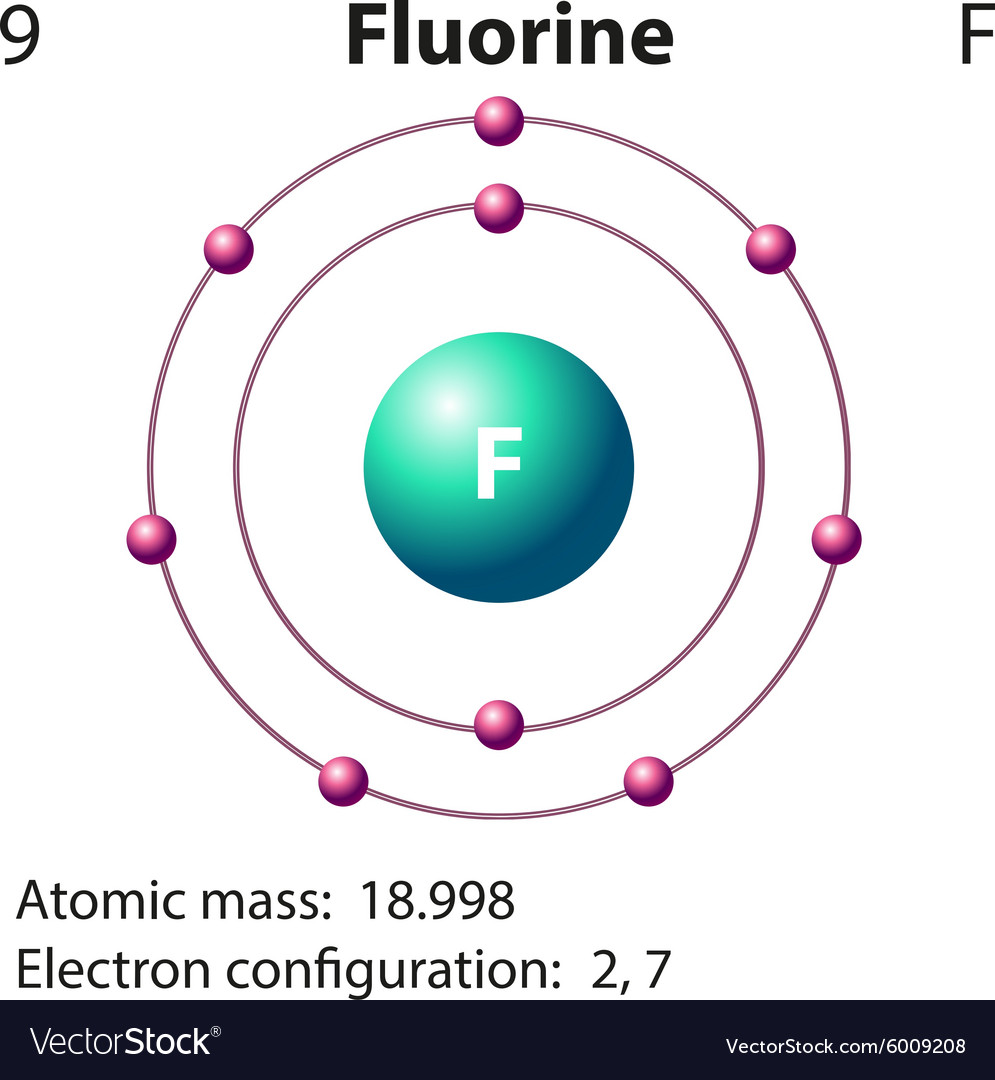
Diagram representation of the element fluorine Vector Image
Knowing this information allows us to set up our Bohr-Rutherford Diagram. Let's create one using Lithium. First determine the number of protons, neutrons and electrons. Lithium. Atomic Number = 3 #p = 3 (since atomic number is 3) Atomic Mass = 7 #n = 4 (since 7 - 3 = 4) #e = 3 (since same as #p) The Bohr-Rutherford Diagram for Lithium would.
Bohr Model Of Fluorine Atom With Proton, Neutron And Electron Royalty
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Fluorine atom with some simple steps. Steps to draw the Bohr Model of Fluorine atom 1. Find the number of protons, electrons, and neutrons in the Fluorine atom

Fluorine atom diagram concept Stock Vector Image & Art Alamy
Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules
Fluorine atom Bohr model stock vector. Illustration of flat 267662015
Bohr's model of the atom can be combined with Rutherford's model in diagrams that summarize the numbers and positions of all three subatomic particles. For example, consider the following diagram for Phosphorous: There are certain rules to follow when drawing these diagrams: A circle is drawn in the center to represent the nucleus of the atom.

Grade 9 BohrRutherford Diagram Fluorine YouTube
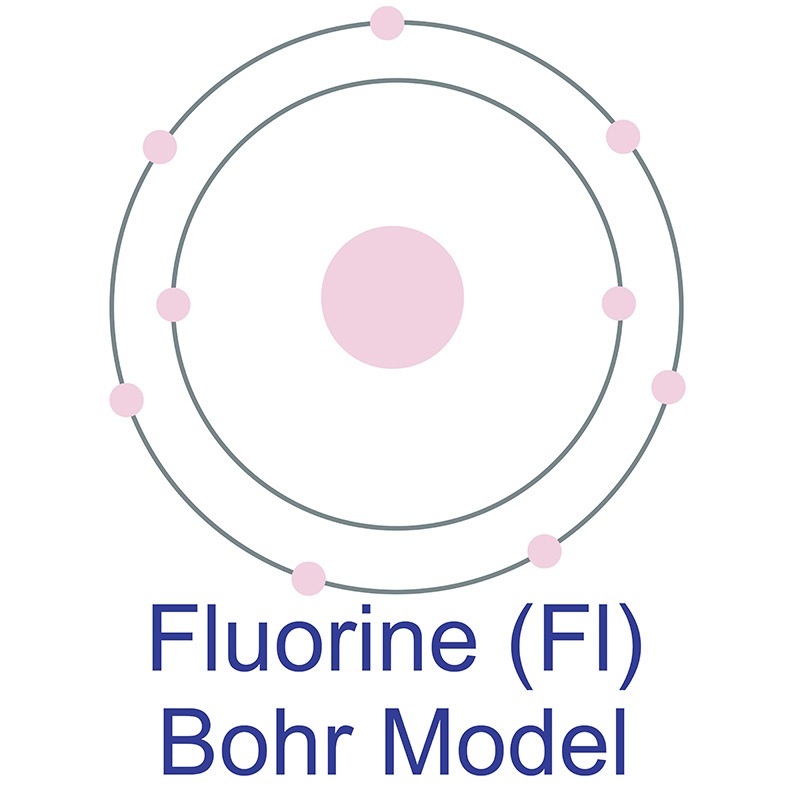
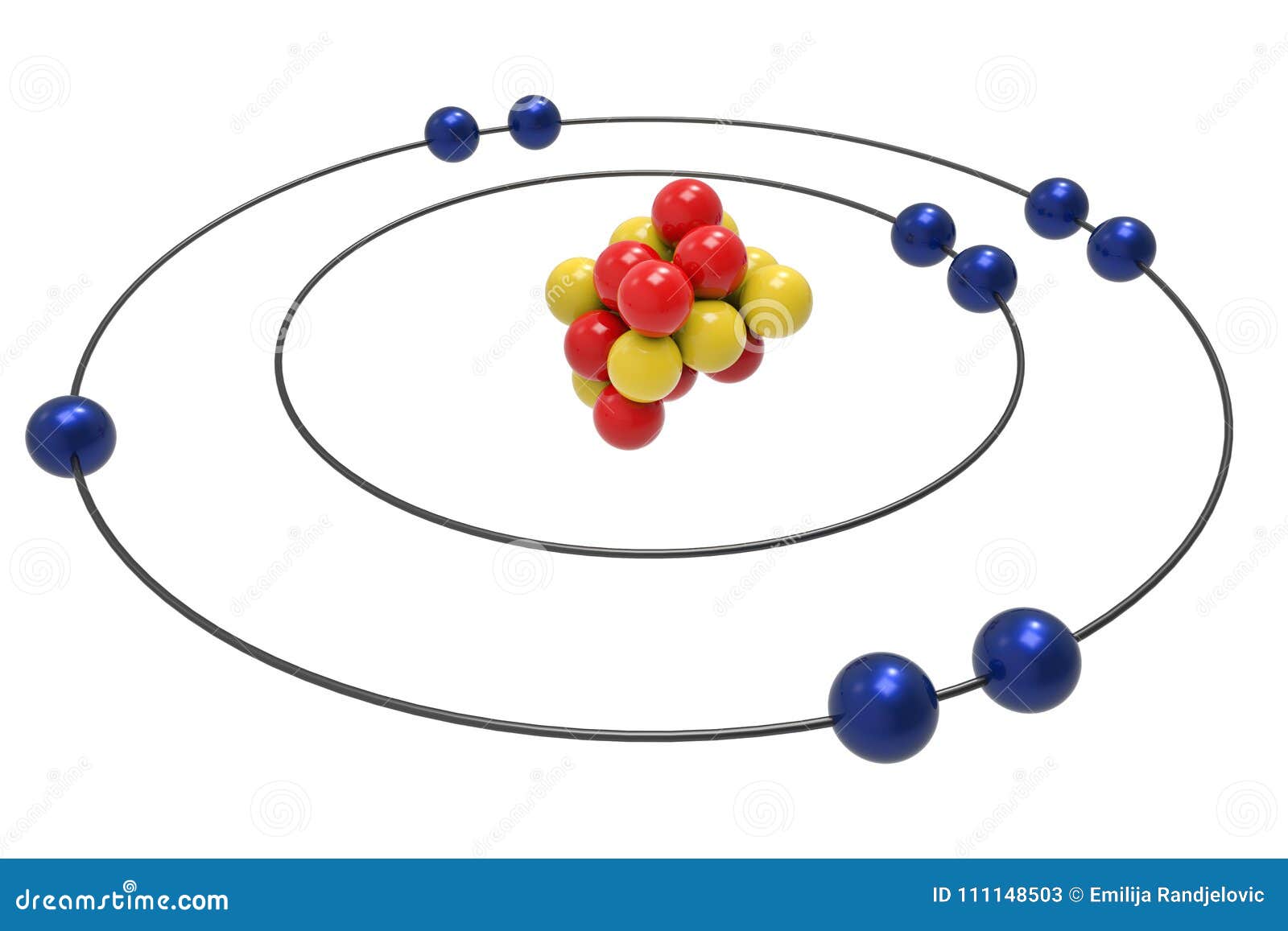
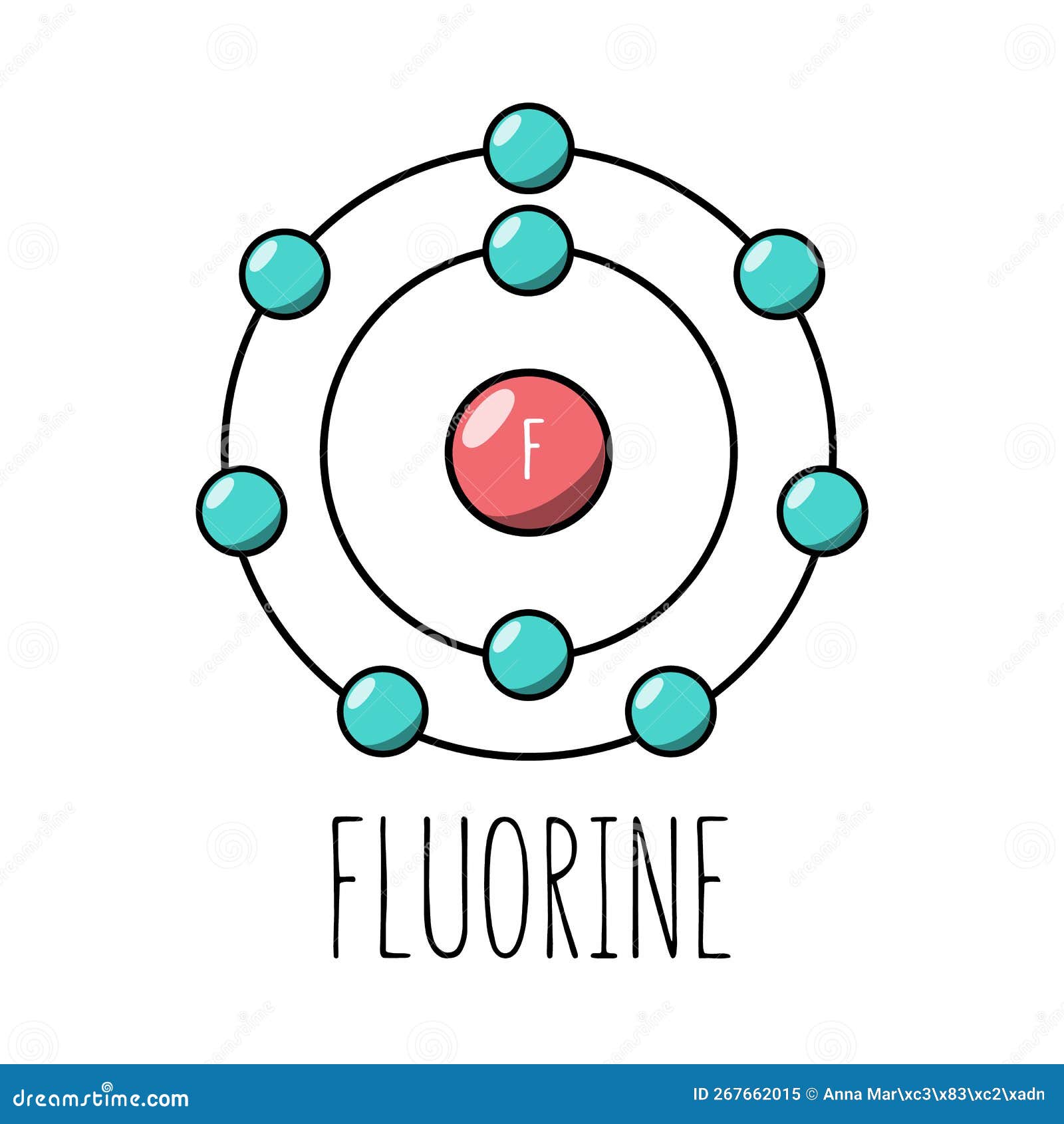
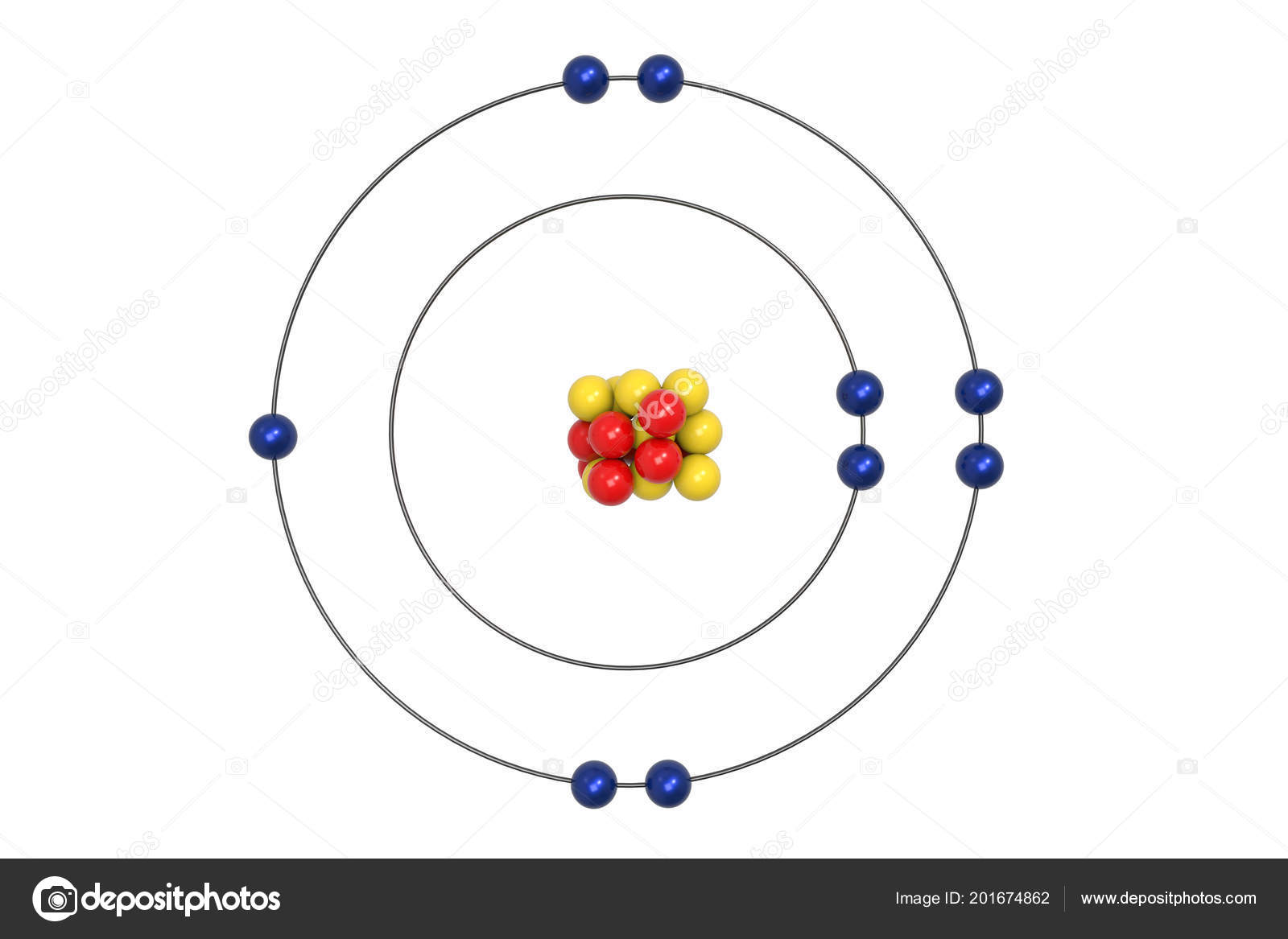
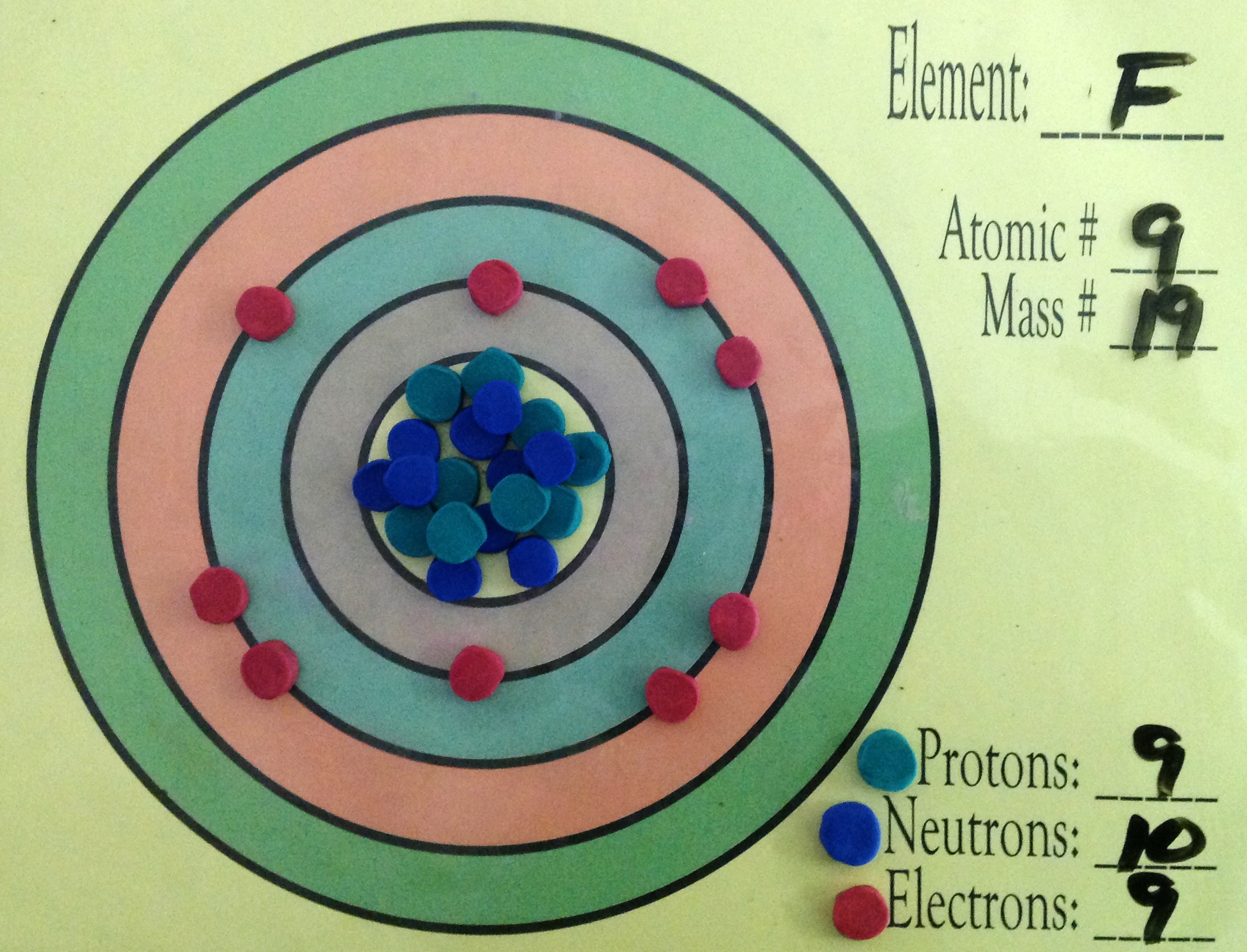
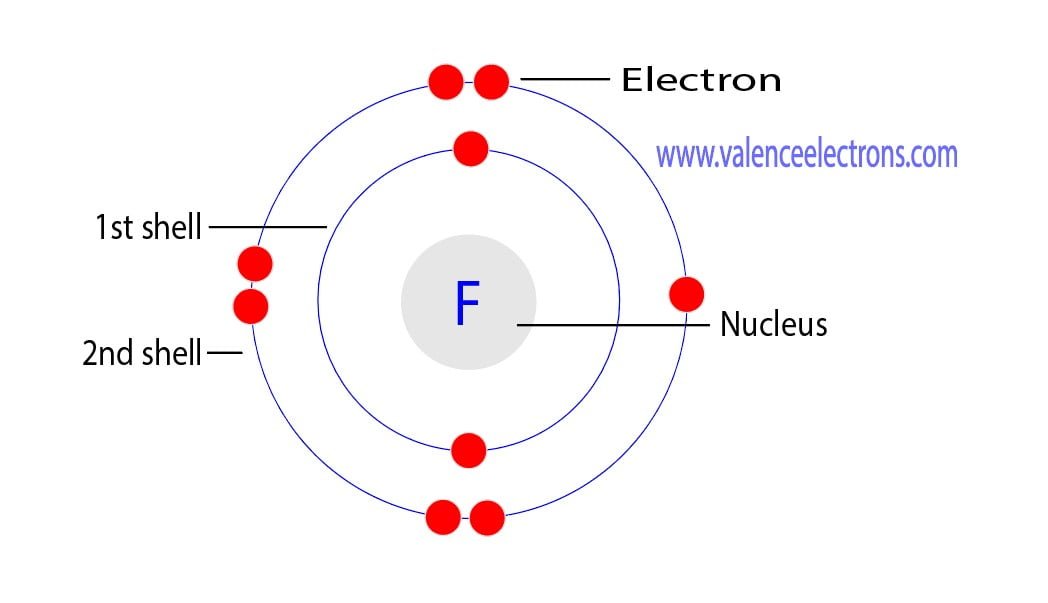
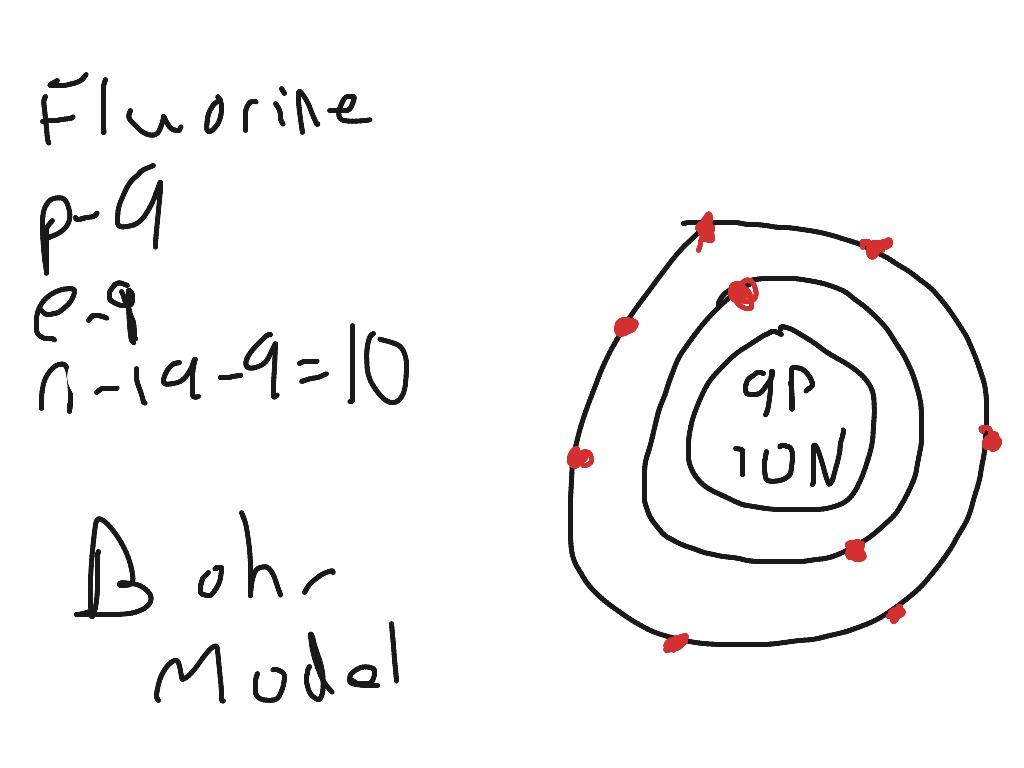
Example 2: Fluorine This is the Bohr -Rutherford Diagram for Fluorine (Atomic Number 9). Fluorine is the ninth element of the Periodic Table. As shown, Fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). Thus, its Atomic Mass is 19. Fluorine also has nine total electrons -- two electrons in Orbit 1, seven electrons in Orbit 2 .

Fluorine Electron Configuration With Full Orbital Diagram
This page contains materials for the session on the atomic models of Rutherford and Bohr. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.

Fluorine Atom Diagram
Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons

6. Draw a BohrRutherford diagram for each of the following molecules
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
Fluorine Atom Bohr Model Proton Neutron Electron Illustration Stock
This is the Bohr-Rutherford Diagram for Fluorine (Atomic Number 9). Fluorine is the ninth element of the Periodic Table. As shown, Fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). Thus, its Atomic Mass is 19. Fluorine also has nine total electrons -- two electrons in Orbit 1, seven electrons in Orbit 2.

Fluorine Molecule Diagram
More. Embed this widget ». Added Aug 1, 2010 by JB1295 in Chemistry. Gives the Lewis Dot structure for any element. Send feedback | Visit Wolfram|Alpha. Lewis Dot Diagram of. Submit. Get the free "Bohr Model Widget" widget for your website, blog, Wordpress, Blogger, or iGoogle.
Fluorine Atom Diagram
In 1913 Niels Bohr combined Rutherford's concept of the nuclear atom with Planck's idea of the quantized nature of the radiative process and developed, from first principles, an atomic model that successfully deals with one-electron structures like the hydrogen atom and one-electron ions such as singly ionized helium, doubly ionized lithium, etc. forming a hydrogen-like or hydrogenic.
Fluorine Atom Diagram
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.
Fluorine Bohr Diagram
In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons.

Fluorine Bohr Model
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)
Fluorine Bohr model Science ShowMe
Schematic diagram of the Rutherford-Bohr atomic model with one orbital electron of mass m e and a finite nucleus of mass M. Both the orbital electron and the nucleus revolve about their common center-of-mass. Full size image. The following relationships will be useful in our calculations.